You are here: / Home
CO₂ GAS HYDRATES FOR SUSTAINABLE ENERGY AND COOLING SOLUTIONS
Federal Ministry for Economic Affairs and Energy
05/2025 - 10/2027
Dr. Joachim Germanus
+49-351-4081-5412
in Bearbeitung
COOGaHyd
The objective of the project is to utilise CO2 gas hydrates for refrigeration and energy technology applications, with a particular focus on enhancing the efficiency of cold storage, thereby contributing to the achievement of the climate targets set out by the Federal Republic of Germany. It is imperative that fundamental investigations are conducted into the improvement of clathrate formation of carbon dioxide in water using suitable promoters and matrices.
Energy efficiency and environmental concerns are progressively gaining significance in the domain of refrigeration applications. It is evident that both consumers and companies favour energy-efficient and sustainable solutions with the objective of reducing energy costs and their ecological footprint. This development is increasing demand and fuelling innovation in the field of energy-efficient and environmentally friendly cooling systems. In conclusion, it is evident that cooling will become increasingly necessary in the future.
The utilisation of CO2 gas hydrates is a promising option for meeting the above-mentioned requirements for efficient cold storage. The use of CO2 gas hydrates (e.g. also to reduce the CO2 content in the atmosphere [1] and thus prevent ocean acidification, but also applications to capture CO2 from flue gas are being discussed [2]) could also help to reduce greenhouse gas emissions and further reduce dependence on fossil fuels. In this context, it is of great interest to examine and investigate the properties, behaviour and potential applications of CO2 gas hydrates in more detail. The aim of the research project is to make a well-founded contribution to the investigation of this interesting substance in relation to refrigeration technology and to develop innovative solutions for sustainable energy and cooling solutions.
Gas hydrates are non-stoichiometric inclusion compounds, also known as clathrate hydrates. They are solid substances with ice-like structures made up of water and gases. They form under high pressures and low temperatures above the melting point of water. In nature, they can be found as methane hydrates in deep sea beds and in permafrost regions. Gas hydrates are stabilised by hydrogen bonds that form cage structures in which gas molecules are trapped. The substance is similar in appearance to ice but, as in the case of CO2 gas hydrate, has a higher density of 1.1 g/cm³. It is therefore heavier than water and does not float on the liquid phase like ice. Gas hydrates are thermodynamically unstable at room temperature and atmospheric pressure, but their decomposition is kinetically inhibited by the cage-like inclusion of the gas component.
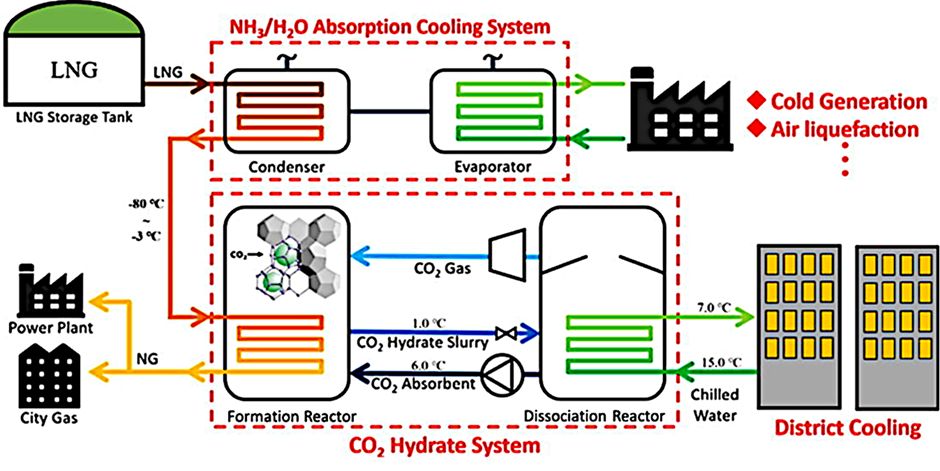
Due to the high decomposition enthalpy or high latent heat of pure CO2 gas hydrate of between 502 and 507 kJ/kg [3] compared to the phase change enthalpy of water/ice at 333 kJ/kg3, CO2 gas hydrate has advantages as a cold storage medium compared to thermal water/ice storage. The CO2 gas hydrate phase change process can take place in a temperature range between 0 °C and 15 °C and at pressures below 15 bar (adjustable as required and therefore unique and advantageous compared to classic phase change materials). One possibility for the sensible utilisation of CO2 gas hydrates for district cooling networks is shown using the example of cold thermal energy recovery from LNG (liquefied natural gas) and is illustrated in Fig. 1. Against the background of a future increase in the use of LNG in Germany, the coupling of various thermal processes would be of great benefit from an energy and ecological point of view.
[1] Nago A, Nieto A.: Natural gas production from methane hydrate deposits using clathrate sequestration: State-of-the-art review and new technical approaches. Journal of Geological Research. 2011:1-6
[2] Seo Y-T, Moudrakovski IL, Ripmeester JA, Lee J-W, Lee H. Efficient recovery of CO2 from flue gas by clathrate hydrate formation in porous silica gels. Environmental Science &Technology. 2005; 39(7):2315-2319
[3] X. Wang et al.: Solar Energy; Vol 211, Nov. 2020, S. 11-30
[4] Choi, S. et al.: Experimental investigation on CO2 hydrate formation/dissociation for cold thermal energy harvest and transportation applications; Applied Energy, Volume 242, 15 May 2019, Pages 1358-1368