You are here: Home / Research and Development
Electrochemical decontamination of electrically conducting surfaces „EDeKo II“
Improvement of sanitary prevention by electrochemical decontamination
Due to the risk of widely spreading illnesses and pandemic diseases, hygiene is becoming more and more important on a global scale. One method to reduce breakouts is prevention by eliminating dangerous contamination of surfaces (decontamination). Under the direction of Dr. Burkholz, the ILK Dresden is doing research on an economic and effective method, the method of electrochemial deconamination of electrically conducting surfaces, to improve and extend existing sanitary measures. The effect of the decontamination method is an electrochemical attack proceeding from the contaminated surface. This could be the surface of medical equipment, for example. By the electrochemical generation of reactive oxygen species (ROS), all attached biological material is destroyed. Even biofilm, which protects itself from chemical detergents by a mucous layer, can be destroyed effectively with the help of this principle.
Field of use
Improvement of existing cleaning, sterilization and decontamination processes, e.g. in medicine, air-conditioning and ventilation engineering, the food industry and cleanroom technology.
Objective
„EDeKo II“ will deal with the generation of reactive oxygen species as well as material stability under increased temperatures, the impact of flow effects and different pressures. Furthermore, new devices will be developed and built, which ensure electrochemical decontamination under application-relevant circumstances.
Procedure
In the previous project „EDeKo“, the generation of reactive oxygen species (ROS) has been optimized on a laboratory scale under set conditions. In „EDeKO II“, the impact of diverse factors on the generation of ROS will be checked, such as a variable electrode gap, an altered relation between anode and cathode area as well as different pressures and temperatures. For the experimental measurement, new electrochemical cells will be designed and built, which create the distance of the electrodes to one another in defined steps variably and ensure different geometries for the anode.
Picture material

The experimental basis for the electrochemical analysis will be the Potentiostat Metrohm Autolab PGSTAT 204, in which temperatures of up to 95°C and pressure of up to 10 bar maximum are possible, including the related measurement and evaluation software (Nova 2.0) as well as different stainless steel pressure containers, e.g. see-through sterilizers, which have been developed at the ILK Dresden.
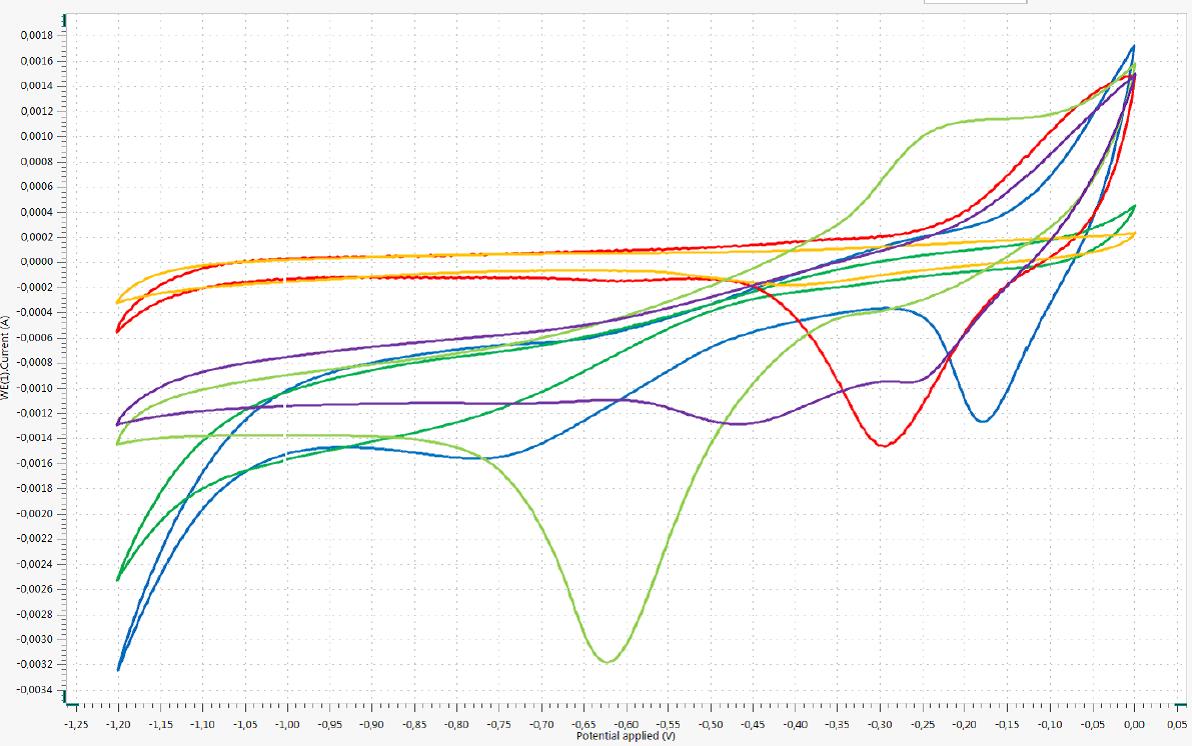
Abbildung: Cyclovoltammogramme einer Cu-Arbeitselektrode gegen die Ag/AgCl-Elektrode mit der in Kaliumphosphat-Puffer (pH 7; blau), Kaliumdihydrogenphosphat-Lösung (pH 4,5; dunkelgrün), Dikaliumhydrogenphosphat-Lösung (pH 9,2; rot), Natriumsulfat-Lösung (pH 5,9; orange), Natriumcitrat-Lösung (pH 8,3; hellgrün) und Natrium-Bicarbonatpuffer (pH 6,5; violett).
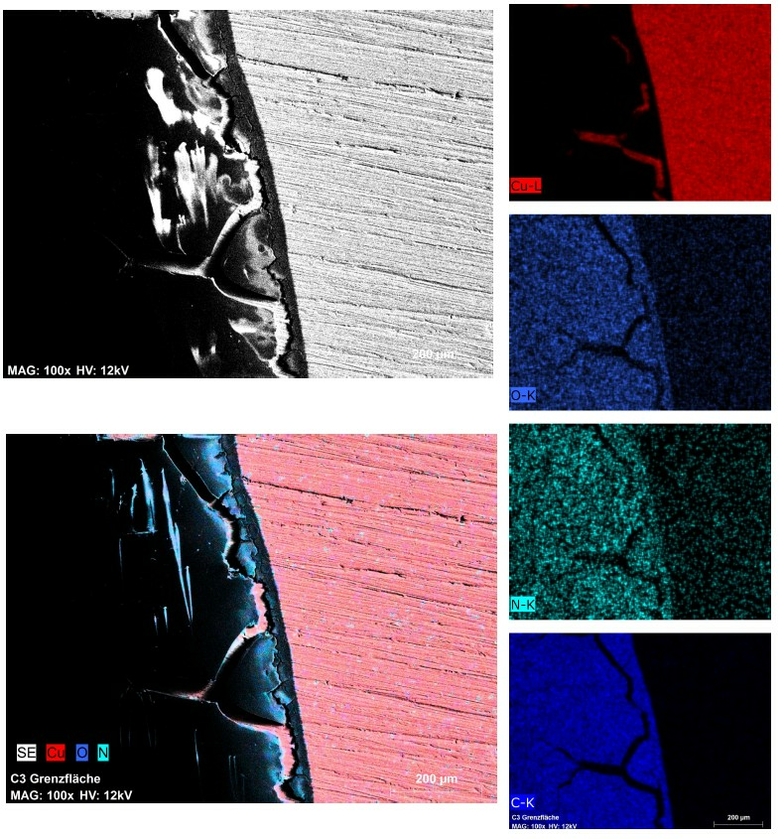
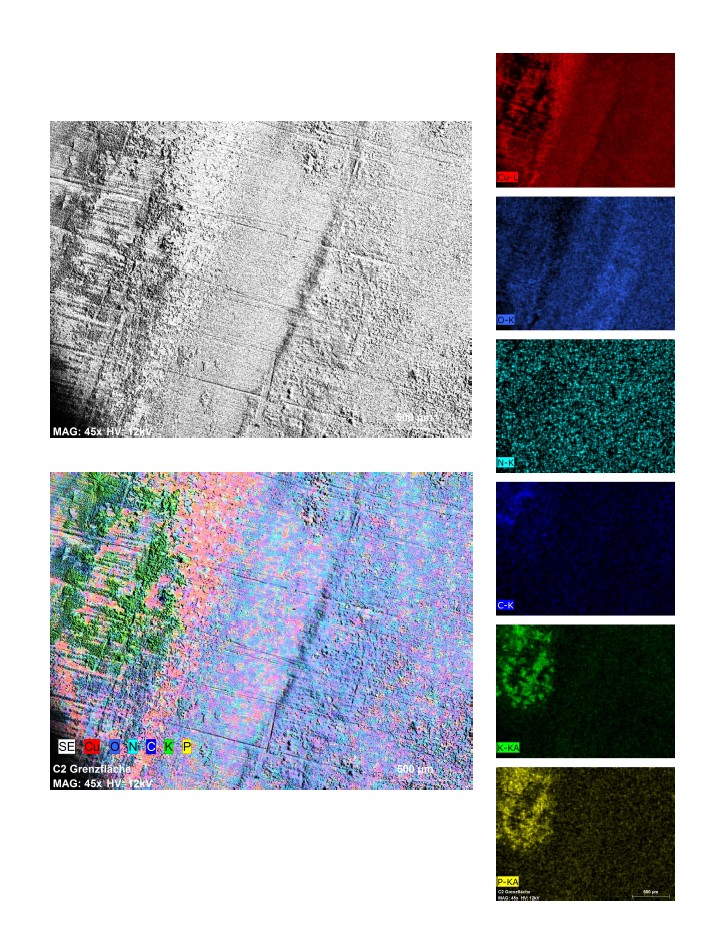
Abbildung: Bild1 Elementverteilung einer mit Protein behandelten Cu-Oberfläche ohne elektrochemische Vorbehandlung. Bild2 Nach elektrochemischer Vorbehandlung.
Related links
https://www.wotech-technical-media.de/womag/ausgabe/2014/05/24_med_burkholz_05j2014/24_med_burkholz_05j2014.php (Link to WoMag-Publikation)
https://publikationen.sulb.uni-saarland.de/handle/20.500.11880/22719 (Dissertation)
T. Burkholz, Elektrochemische Dekontamination elektrisch leitfähiger Oberflächen (EDeKo) - Abschlussbericht, EURONORM GmbH, VF160045 (2019).
C. Jacob, G. Kirsch, A. Slusarenko, P.G. Winyard, T. Burkholz (eds.), Recent Advances in Redox Active Plant and Microbial Products: From basic chemistry to widespread applications in Medicine and Agriculture, Springer Science, ISBN: 978-9401789523 (2015).
T. Burkholz, C. Jacob, A word on redox activity, in: C. Jacob, G. Kirsch, A. Slusarenko, P.G. Winyard, T. Burkholz (eds.), Recent Advances in Redox Active Plant and Microbial Products: From basic chemistry to widespread applications in Medicine and Agriculture, Springer Science (2015).
U.M. Viswanathan, T. Burkholz, C. Jacob, Electrochemistry at the edge of reason: Chalcogen-based redox systems in biochemistry and drug design, Zeitschrift für Physikalische Chemie, 227(5), 691-706 (2013).
E. Domínguez Álvarez, U.M. Viswanathan, T. Burkholz, K. Khairan, C. Jacob, Bio-Electrochemistry and Chalcogens, Chapter 7, in M. Schlesinger (ed.), Applications of Electrochemistry in Medicine, Modern Aspects of Electrochemistry, 56, 249-282 (2013).
Your Request
Further Projects - Research and Development
Characterisation of Superconductors in Hydrogen Atmosphere
Are superconductors really compatible with hydrogen?
Development of a Cryogenic Magnetic Air Separation Unit
Oxygen Enrichment by Applied Cryogenic Magnetohydrodynamics